
Joint Declaration, 15 May 2022
We are an informal coalition of civil society organisations that believes a robust global framework for the protection of intellectual property (IP) rights plays a key role in ensuring the world is prepared for future pandemics.
In the current pandemic, the IP system underpinned the development of multiple new safe and effective vaccines in record time and the manufacturing of billions of doses, saving hundreds of millions of lives. The ability of the private sector to innovate, manufacture, and distribute has brought life-saving therapeutics and vaccines to epidemics in the past, and it will no doubt play a key role in future pandemics.
Nevertheless, there has been a call by some member states to dilute or remove IP rights in the Pandemic Prevention, Preparedness and Response (PPR) Instrument in the early stages of discussion at the World Health Organization (WHO). The claim is that equitable access can only be achieved if developing countries manufacture vaccines locally, using proprietary technology and know-how forcibly appropriated from innovators.
Removing or weakening IP rights for pandemic vaccines and therapeutics would be highly counterproductive. It would undermine the incentive to invest in research and development (R&D) into new technologies and treatments. Equally as important, it would destroy the international manufacturing collaborations and partnerships that have proved indispensable to saving millions of lives in the current pandemic.
Rather than limiting IP rights in pandemic situations, a more sensible approach is for governments to focus on concrete measures to accelerate equitable access to vaccines and therapeutics, in particular harmonising regulation and dismantling trade barriers.
The protection and strengthening of the R&D environment, including the robust protection of IP rights, should be at the heart of future pandemic planning to ensure the development of multiple vaccine and therapeutic options, and their widespread and rapid availability.
1. IP rights have been crucial to developing safe and effective vaccines for the current pandemic. They are also crucial for future pandemics.
In December 2019, COVID-19 was an unknown disease. Now there are ten COVID-19 vaccines authorised under emergency use listing by the WHO, with over 60 vaccine candidates in late stage clinical trials (Phase 3 or 4) or pending regulatory review.
This triumph of innovation has been underpinned by intellectual property rights. In the early days of the pandemic the IP system encouraged the rapid establishment of dozens of partnerships around COVID-19, with even commercial rivals prepared to cooperate and share proprietary intellectual resources such as compound libraries. One of the most notable partnerships of the pandemic, between Pfizer and BioNTech, resulted in a vaccine that has been a mainstay of national vaccination programmes all over the world.
Such partnerships depend on sharing large volumes of commercially sensitive material and would not occur without the legal certainty provided by IP rights. Protection of IP allows the safe exchange of proprietary knowledge at all stages of the drug development cycle.
In the early stages of R&D, the public disclosure inherent to patent rights enables drug developers to identify partners with the right intellectual assets such as know-how, platforms, compounds and technical expertise. Without patents most of this valuable proprietary knowledge would be kept hidden as trade secrets, making it impossible for researchers to know what is out there.
Additionally, the existence of laws protecting intellectual property helps rights-holders make the decision to collaborate in the first place. By allaying concerns about confidentiality, IP rights enable companies to open their compound libraries, and to share platform technology and know-how without worrying they are going to sacrifice their wider business objectives or lose control of their valuable assets.
For instance, rights holders might contribute IP that is useful for entirely different diseases to COVID-19 R&D. There are dozens of medicines that have been screened for efficacy against Covid or its clinical sequelae, some of which are patent protected for their original indication.
IP rights also enabled collaboration between regulatory agencies including pre-purchase agreements which have proven to be crucial to pandemic preparedness.
Likewise, IP enables risk-taking in R&D efforts, including those which led to the first mRNA vaccines. After decades of failed R&D attempts, these risky endeavours paid off and offer the potential for rapid solutions for future pandemics.
If IP rights are uncertain in a pandemic situation, such risk-taking, voluntary collaboration and information sharing would be far less likely. COVID-19 has shown the value of partnership, underpinned by IP rights, in rapid and effective pandemic R&D. To ensure the strongest environment for innovation, strong protection of IP rights should be at centre of any future treaty to prepare for pandemics.
2. Pandemics require rapid scale up of mass vaccine manufacturing. IP makes it happen.
From a standing start in 2020, the scale-up of COVID-19 vaccine manufacturing has been remarkable. There were a total of 12 billion doses manufactured by December 2021, enough to vaccinate the world’s eligible population if evenly distributed. COVAX expects enough doses in 2022 to meet its commitments to participating countries. Currently, vaccine supply far exceeds the global capacity to administer safe and effective COVID-19 vaccines in an equitable manner, yet a range of non-IP challenges relating to public health infrastructure, vaccine hesitancy and other problems mean that vaccine roll-outs have been slow.
IP rights have been fundamental to this massive, rapid scale-up of vaccine manufacturing by allowing innovator companies to enter into manufacturing partnerships all over the world. For example, Pfizer/BioNtech and Johnson & Johnson have each partnered with their rival Merck to increase production of their cutting-edge vaccines. Partnerships span the entire manufacturing value chain, existing in every continent and have been rapidly rising in number (Figure 1).
Given the novelty and complexity of the technology platforms used to make COVID-19 vaccines, these partnerships require the active transfer of technology through teaching and physical presence by key personnel. IP protections allow this to take place by giving the innovator trust and confidence that valuable information can be shared without risk.
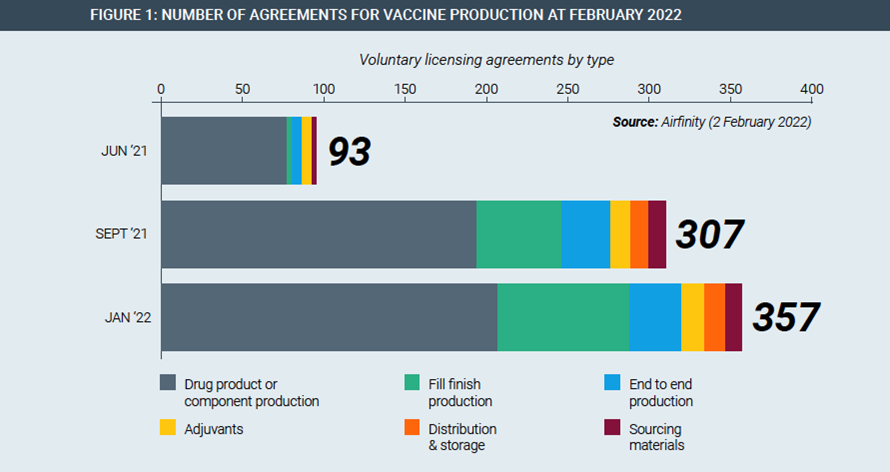
In each of these partnerships, trade secrets and other proprietary information is protected with both contractual agreements and the existence of laws protecting IP rights. An IP waiver, or limitations on IP in a pandemic preparedness instrument, would require innovators to reveal their know-how under threat of legal force, with very different consequences than voluntary cooperation.
First, forced transfers would likely be contested both in law and fact, as innovators would not want to divert their most knowledgeable and busy employees during a global crisis. Transferring this know-how could take many months, followed by further delays while regulators scrutinise any new manufacturing facilities and their products for quality standards. Pandemic-related travel restrictions would make this process even more difficult.
Moreover, forcing the disclosure of a trade secret destroys it, as it is no longer secret. Secrecy is the fundamental legal and practical requirement for the existence of a trade secret. When a patent owner is compelled to license a patent, it still owns the patent and can receive a reasonable royalty. By contrast, forced disclosure destroys a trade secret and its value.
If governments were to force technology transfer it would therefore represent a fundamental assault on private property rights and contract law which would have disastrous economic implications beyond the pandemic. At the very least it could destroy many small biopharmaceutical companies whose main assets are the IP rights they hold around small numbers of technologies.
Voluntary technology transfer based on cooperation, appropriate training and resource sharing is therefore key to establishing additional capacity. This cannot be achieved without strong IP rights.
3. Weakening IP rights would leave the world dependent on unproven vaccine R&D and manufacturing models
If IP rights are weakened in any national or multilateral forum, few private sector companies would be willing to engage in R&D or mobilise the capital needed to scale up mass manufacturing. This would leave the world reliant on alternative open source or IP-free models of drug and vaccine development. Current experience is not promising.
While a few examples have advanced towards market authorisation, their development has been too slow for a pandemic situation:
- The University of Helsinki vaccine has been unable to secure the necessary funding for clinical trials for its IP-free vaccine, and there are currently no details about the preclinical studies available publicly.
- Corbevax, a patent-free vaccine developed by a consortium including the Texas Children’s Hospital has been authorised for use in India, but neither its developers nor the Indian regulatory authorities have released any peer-reviewed data on the vaccine’s efficacy and none on any clinical trial.
If successful, IP-free vaccines could prove useful additions to the pandemic preparedness arsenal. But the difficulties such patent-free models face in marshalling the capital and expertise to rapidly scale-up global production make them unreliable in a pandemic situation where speed is of the essence.
By contrast, vaccines that have leveraged IP rights have moved quickly through clinical development, regulatory authorization, and into mass manufacture and distribution.
4. Harmonising regulation would speed up vaccine availability
During the COVID-19 pandemic a lack of regulatory harmonization led to very different availability timelines in different countries. Emergency approval pathways for pandemic health technologies are available, but researchers reviewing the regulatory landscape in the early stages of the pandemic found at least 51 pathways to various types of accelerated vaccine approval in a group of 24 countries. This confusing picture made it difficult for manufacturers to prioritise and spread their resources across different regulators, all of which have slightly different requirements.
If every country were to carry out its own review of a vaccine, it would force the manufacturer to go through 190 different regulatory processes, according to Richard Hatchett, chief executive officer of the Coalition for Epidemic Preparedness Innovations.
To accelerate access to pandemic vaccines and therapeutics, efforts to harmonise regulatory requirements should therefore be at the heart of any new pandemic PPR instrument.
5. Countries should not disrupt vaccine and therapeutic supply chains with trade barriers.
During the COVID-19 pandemic trade barriers have disrupted vaccine availability in both developed and developing countries. Export restrictions imposed by India in April 2021 meant COVAX was around 190 million doses behind schedule by June 2021, massively delaying the response to the pandemic in low and middle income countries. Given that vaccine manufacturing relies on globally distributed supply chains, export restrictions must never again happen in future pandemics.
Meanwhile, a host of issues related to trade facilitation can delay access. For example, inefficient cooperation between border agencies or reliance on outdated and paper-based processes can cause vaccines and pharmaceutical products with short shelf lives to be held up at borders for weeks in expensive storage facilities. Different certification requirements for the various components of vaccine kits can add to administrative delays.
Governments must credibly and effectively commit to keeping global supply chains for vaccines and disease treatments flowing freely during pandemics. Such a commitment should be legally enforceable, for example via a new mechanism at the World Trade Organisation. (*)
This declaration is supported by:
- Adam Smith Centre, Singapore
- Alternate Solutions Institute, Pakistan
- Austrian Economic Centre
- Centre for Development Policy and Practice, India
- Competere – Policies for Sustainable Development, Italy
- Centre for Indonesian Policy Studies
- Consumer Choice Centre, Brussels
- Fundación Eleutera, Honduras
- Fundación IDEA, Mexico
- Galen Centre for Health and Social Policy, Malaysia
- Geneva Network, United Kingdom
- Hayek Institute, Austria
- I-Com, Institute for Competitiveness, Italy
- Imani Centre for Policy and Education, Ghana
- Instituto de Libre Empresa, Peru
- Instituto de Ciencia Politica, Colombia
- Istituto Bruno Leoni, Italy
- Information Technology and Innovation Foundation, United States
- Laboratory of Industrial and Energy Economics,
- National Technical University of Athens, Greece
- Libertad y Desarrollo, Chile
- Libertad y Progreso, Argentina
- Macdonald Laurier Institute, Canada
- Paramadina Public Policy Institute, Indonesia
- Property Rights Alliance, United States


